

For example, in order to convert water to steam, energy must be added to the water, so for that process, the energy released to the surrounding area is negative. Calculate the molar heat(enthalpy) of neutralization of KOH. Yes, the energy released can be negative for processes that require additional energy to occur. Total heat due to both temperature and moisture can be expressed in SI units as: ht q dh (3) where ht total heat (kW) q air volume flow (m3/s) density of air (1. We go from an average initial temperature of 22.3 oC to a maximum of 29.2 C. Usually, the heat capacity equation expressed at constant pressure (Cp) and volume (Cv) and energy unit is used for its calculation in physics or chemistry. The change in thermal energy due to temperature changes is calculated using this equation: change in thermal energy mass ×.
#Heat equation chemistry calculator how to#
The lost mass was converted directly to energy. Specific heat calculator capacity and chemistry tutorial you how to calculate 6 steps with pictures wikihow equation tessshlo enthalpy what is the lessons examples step by solutions problems calculations calorimetry molecular formulas nomenclature using enthalpies of reaction Specific Heat Calculator Heat Capacity And Specific Chemistry Tutorial You How To Calculate Specific Heat 6 Steps With. During fission, an atom splits into two smaller nuclei that have less mass combined than the initial atom. The process of fission releases energy through the conversion of mass into energy. For example, in radioactive materials, the matter is converted to energy during radioactive decay.
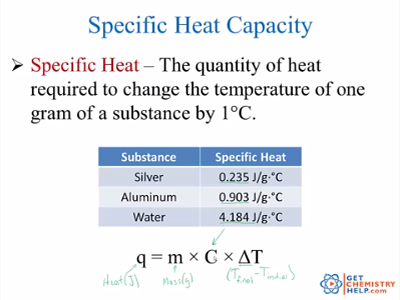
Can energy be released when matter changes?Įnergy can be released when matter changes in instances in which the net amount of matter is reduced from the process. There are many sample equations in this chemical equation balance calculator so that you can practice and balance equations. Sample Calculation: Heat Capacity of Calorimeter. For example, a combustion process will heat up the air around the combustion and increase its temperature. Chemical reactions involving the transfer of heat are carried out in devices called calorimeters. Next, determine the change in temperature.Ĭalculate the change in temperature that happens during the process.Ĭalculate an energy release using the formula above.Įnergy is released in any chemical or physical process that creates a net positive change in temperature to the surrounding area of that process.Calculate or determine the specific heat capacity.


 0 kommentar(er)
0 kommentar(er)
